We are pleased to announce our Crowdfunding Campaign to raise funds for a new study on the Gupta Program treatment for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), Fibromyalgia or MCS.
This independent study will be in association with a well-known independent clinic and research center that we are currently in talks with.
Purpose Of The New Study
To conduct a randomized, Phase 2 or Phase 3 clinical trial of the Gupta Program in order to offer clear insight into the potential contribution of this method to evidence-based integrative medical strategies aimed at the treatment of chronic conditions such as ME/CFS & Fibromyalgia, as well as MCS/CIRS/Mold Illnesses.
- To test the efficacy of a novel and cutting-edge treatment known as the Gupta Program.
- It will also help us learn about the most effective delivery of the program.
- How we can improve the treatment for the millions of people around the world who suffer from these conditions
We Are Already Well On Our Way To Our Target But We Need Your Help
Just think of the difference we can make together by definitively showing how effective Amygdala & Insula Retraining (AIR) can be at treating these conditions. Together we can help thousands more reclaim their Health and Happiness.

Crowdfunding Goal

We are so confident in the expected benefits of this approach that the Gupta Program will contribute $15,000 towards the study as a starting point.
How You Can Help And Get involved
We are open to obtaining funding from a variety of sources from crowdsourcing to pursuing conventional avenues of grant support.

Here Is How You Can Get Involved
- Please kindly pass on this information to others who might be interested.
- Share this page on Social Media by clicking the share buttons
- If you have suggestions for sources of grant funding that we should apply to, please get in contact with us at info@guptaprogram.com

If You Are In A Position To Financially Contribute
The current estimate is that the study will cost $200,000. This cost-estimate has been derived by employing various cost-cutting measures and automation of data collection to reduce costs. The study offers good value, in that, it offers a high-quality, gold-standard test of the effectiveness of the program in 150 patients for a cost that is substantially less than that of typical randomized, controlled trials of this type.
Overview Of The Study
The study’s medical proposal is currently being finalised. In the meantime, here is a high-level summary of how the study will work:
Background
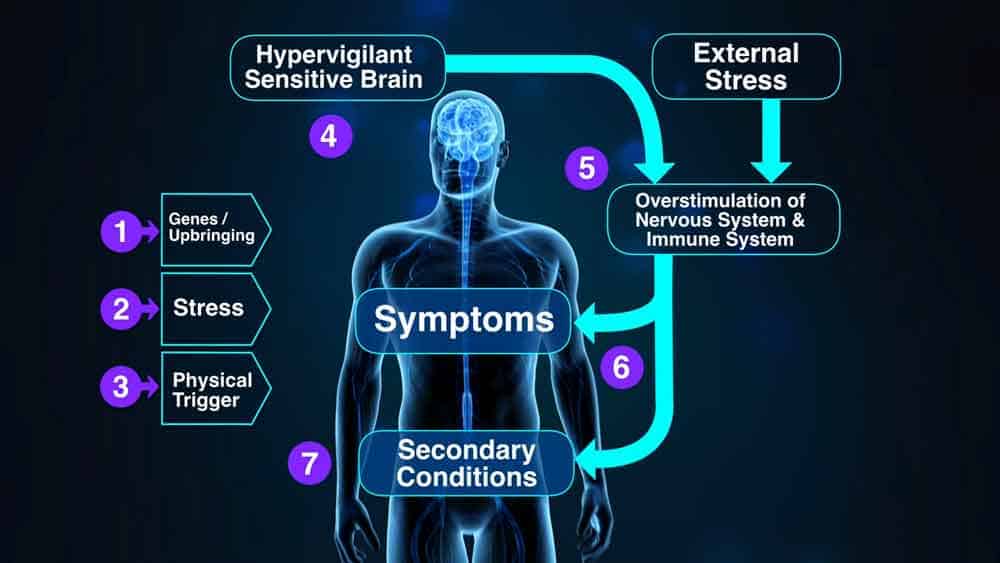
The purpose of this pilot study is to gather preliminary data on the efficacy and feasibility of a mind-body practice termed “Amygdala Retraining Techniques (ART)” on fatigue symptoms and quality of life in patients with Chronic Fatigue Syndrome (CFS). CFS is an incapacitating disorder characterized by profound fatigue, muscle pain, impaired memory, insomnia, and post-exertional malaise.
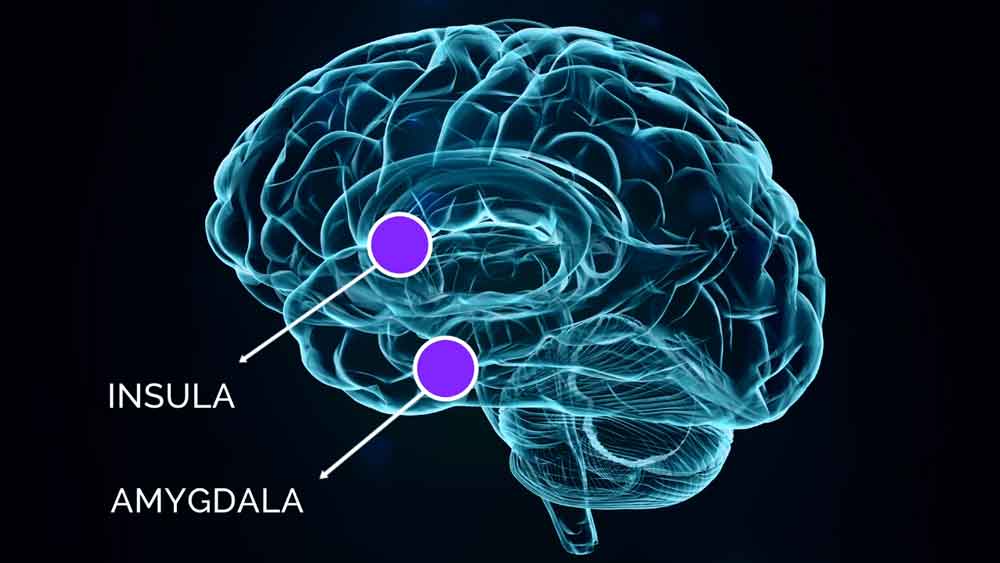
Current literature points to a centrally sensitized state in CFS. Amygdala Retraining Techniques are hypothesised to strengthen neurological inhibitory mechanisms in areas of the prefrontal cortex, insula and anterior cingulate. This helps to reduce hyper-stimulation of the autonomic nervous system by the amygdala and the insula.
There are also aspects of the immune system which are hypothesised to be restimulated by the insula and other structures due to conditioning effects, causing acute symptomatology, as well as allowing opportunistic infections to flourish. The Gupta Program is hypothesised to help retrain the insula, the amygdala, and other associated brain structures by the reinforcement of inhibitory mechanisms, so that the immune system and the autonomic nervous system can return to a normal state of homeostasis.
Overview
This single-blind, randomized, controlled study seeks to gather data on the efficacy of the Gupta Program on symptoms in participants with CFS compared to a standard treatment. Quality of life-related variables (sleep, mood and quality of life) domains will also be measured.
Study Subjects
150 Participants with a confirmed diagnosis of CFS will be recruited to this 6-month study. The patients will be recruited through an established clinic.
Procedures
Participants who consent to the study will be equally and randomly allocated to one of three groups:
1. Active Group: The Gupta Program
2. Primary Control: Standard Medical Interventions carried out at the Fatigue Consultation Clinic
3. Secondary Control: Cognitive Behavioural Therapy (CBT)
1. Active Group: The Gupta Program

The active group will attend a 2-day training course (4 hours per day with rest breaks), where they will learn the key tools and techniques needed for this practice. The participants will then be sent home with an online study program that explains and reinforces the Gupta Program. The main practices in the Gupta Program include specialised neural cognitive restructuring techniques. They are supported by secondary supporting mindfulness-based meditation, alternate nostril breathing and Neuro-Linguistic Programming techniques.
- The participants will be asked to practice the intervention for a minimum of 45 minutes daily up to a maximum of 90 minutes daily. The daily practices include a few minutes of alternate nostril breathing in the morning followed by a simple 20-minute mindfulness meditation practice.
- They will have the option of four meditative practices to pick from a mindfulness meditation practice that cultivates breath awareness or a mindfulness meditation practice that cultivates body awareness.
- Two further guided meditations are also provided. In addition to this, throughout the day they will practice the main “amygdala and insula retraining” techniques to interrupt negative signals to retrain the amygdala and insula’s hypothesized responses.
- These main retraining techniques will take only on average around 20-30 seconds to enact each time. The participants will also be encouraged to take a 20-minute evening meditation if they can make time for it. Again, they will have the option to pick from the four meditative practices above.
- The participants will receive scheduled phone calls or face to face sessions with a trained practitioner for 6 months to help them with obstacles they come across in the practice of the Gupta Program and to tailor the training to them.
- In addition, participants will have timely access to the study investigators for any immediate questions or challenges that arise with the practice
2. Primary Control: Standard Medical Interventions carried out at the Fatigue Consultation Clinic

Medical interventions will be delivered in this control group, based on individual needs. These may, for example, include treatment of viruses and bacterial infections, orthostatic intolerance, or Anti-depressant, anti-anxiety and anti-convulsant medications.
3. Secondary Control: e.g. CBT

This control group will engage in a control group such as a CBT program with a qualified CBT therapist. The number of hours of face time will be similar to the active group.
Data Collection
Assessments will be made at baseline, 3 months, 6 months, 9 months and one year using validated questionnaires assessing: For example, general health (SF36), fatigue (MDFI), mental health (PHQ-9) and sleep quality and problems (Epworth Sleep Scale). In addition, the participants will keep a daily practice log where they record the total time spent daily in the practice of ART and any specific difficulties they encountered in the practice of the program. We would also look to qualified biomedical assessments where possible pre and post treatment.
Inclusion criteria
Age 18 and older, men or women who meet CDC criteria for CFS.
Exclusion criteria
Hypo and hyperthyroidism, untreated | Hypo and hyperparathyroidism, untreated | Adrenal disorders, untreated | Diabetes | Multiple sclerosis | Hepatitis | Cancer (active) | Depression, untreated | Chronic steroid use | Acute inflammatory rheumatological conditions | Obstructive Sleep apnea | Narcolepsy | Pain, uncontrolled as indicated by VAS score of 5 or greater
Potential Risks: This is a set of cognitive mind-body practices aimed at decreasing the stress response and retraining the higher cortical centers. We anticipate no risks to subjects based on Gupta’s 20 years of clinical experience with these techniques.
Gender/Minority Mix: This study will be available to all eligible patients, regardless of race or ethnic origin
Details Of The Research Team
We have assembled a team of experienced researchers who are keen to further independently test the efficacy of the treatment.
Research Site: We are currently in talks with a few sites to establish and finalise a good fit for the study.
Lead Researcher: Dr Loren Toussaint, Associate Professor, Luther College, Iowa, USA. Dr Toussaint will be involved in writing the study protocol, database management, blind assessment and paper submission.

Here Are Some FAQs
The donation that you make will be ring-fenced, and only used towards a study on the efficacy of the Gupta Program. If the study does not go ahead for any reason, we will return the money back to your credit card or Paypal account. The final study details may change from the details on this page, based on IRB submission and other factors.
You will be kept informed of our fundraising campaign, and the timelines for the study.
You can donate in USD on this page, using a Paypal account or credit or debit card.
Please contact us on info@guptaprogram.com and we can discuss your sponsorship of our study.
If You Would Like To Donate Towards Our Target, Please Do So Here
We are very grateful for any contributions that we receive. Once you contribute, you will be added to a list of sponsors of the Gupta Program, and will be kept informed of our progress.
If you wish to donate, you can click on one of the buttons below to make a Paypal or Credit Card payment towards the campaign.
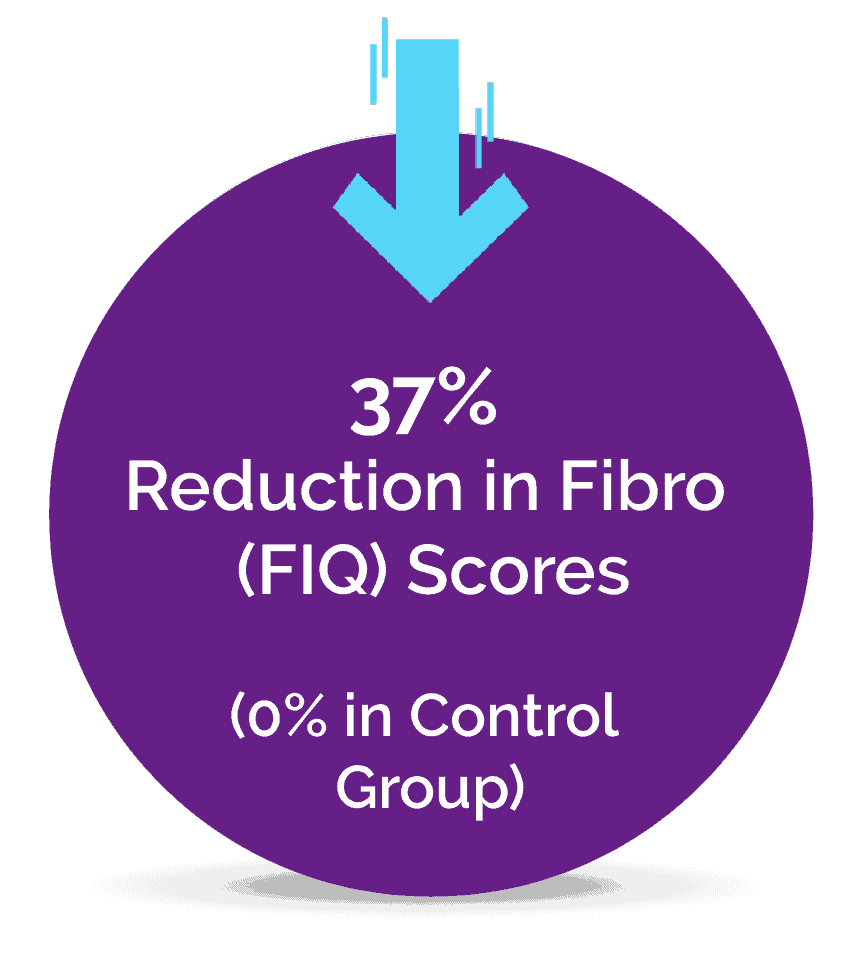
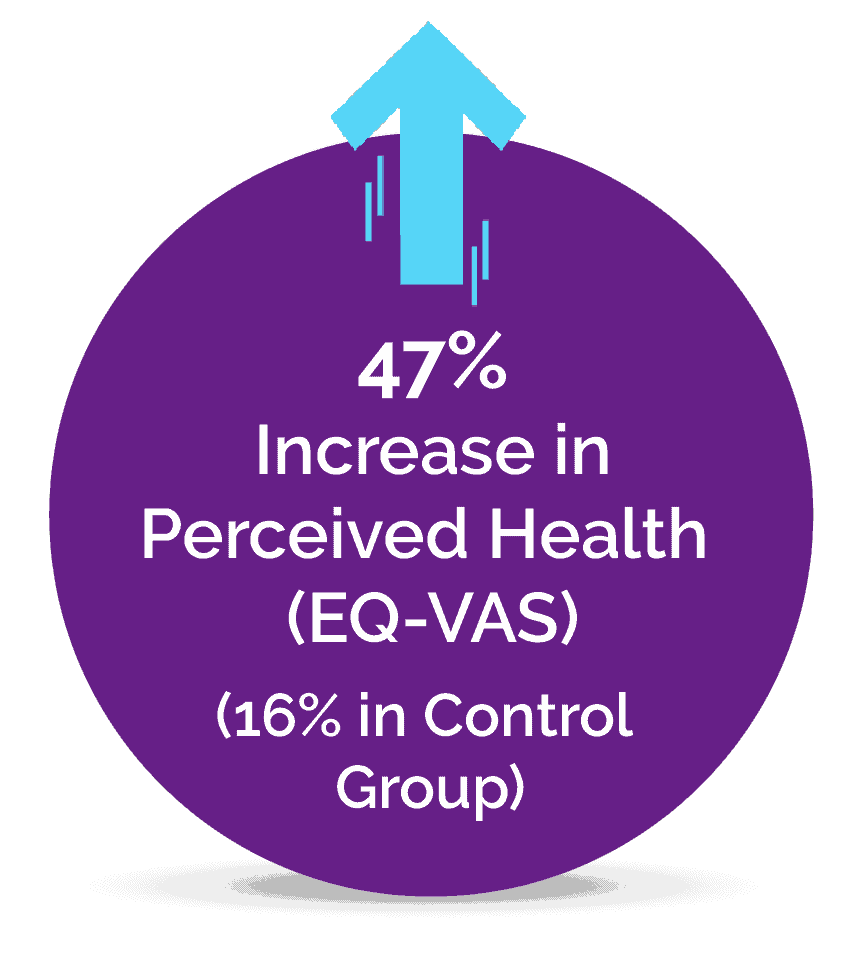
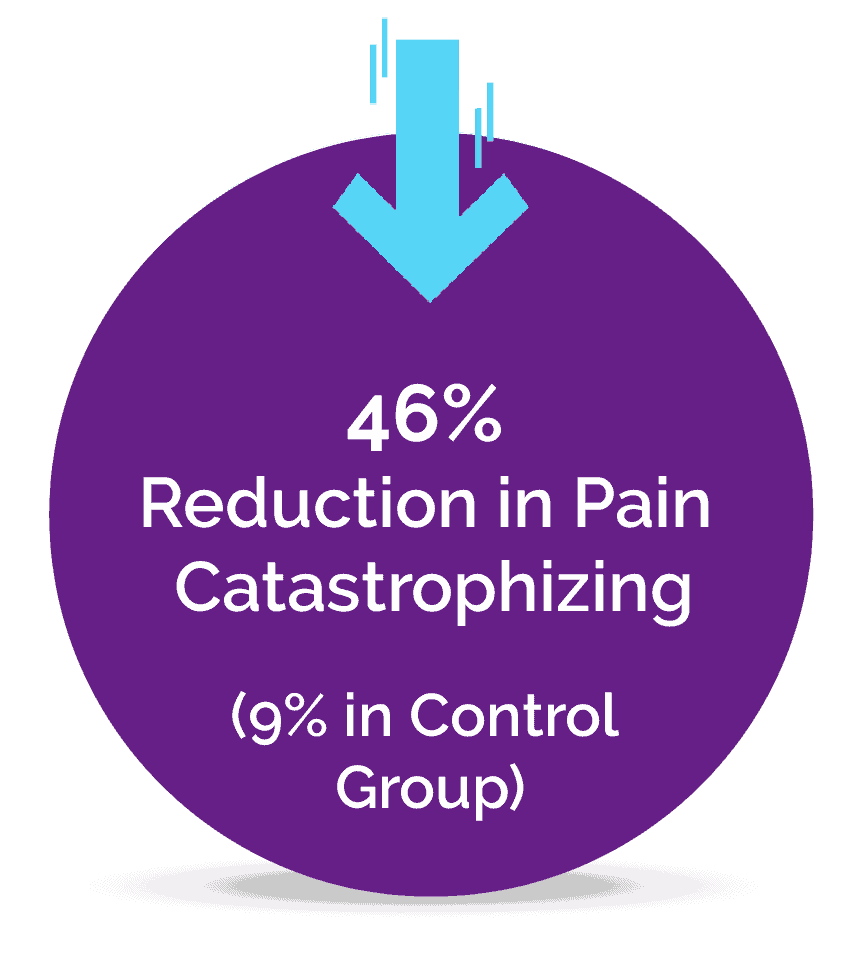
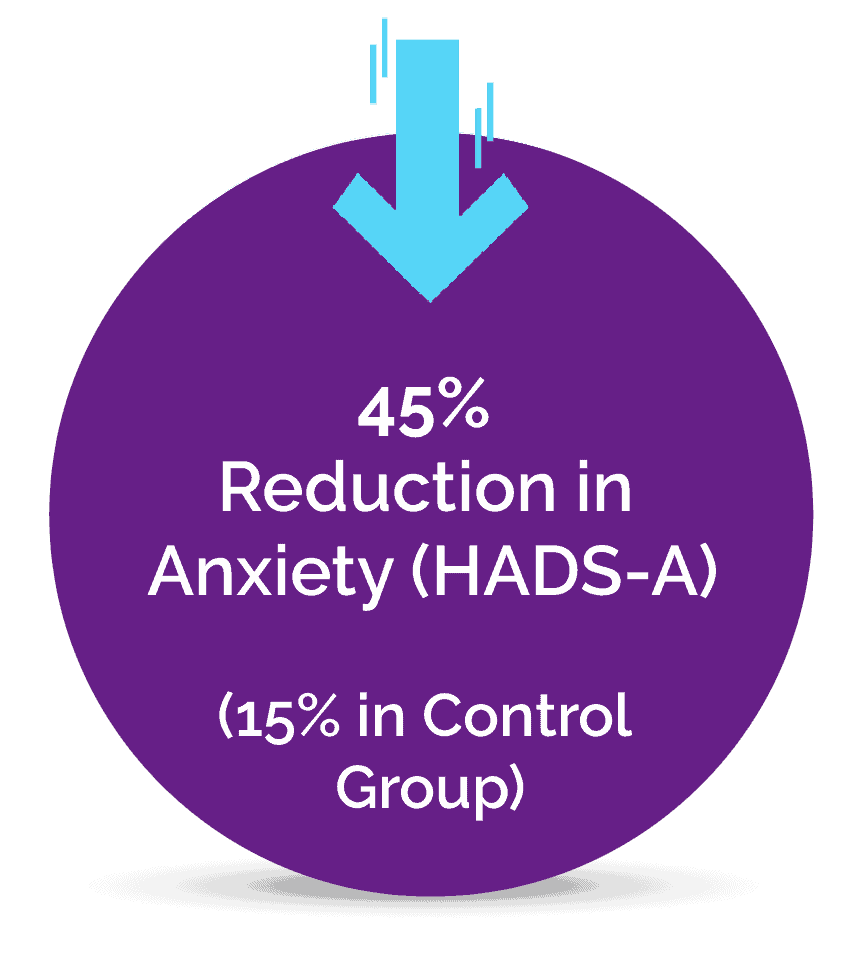
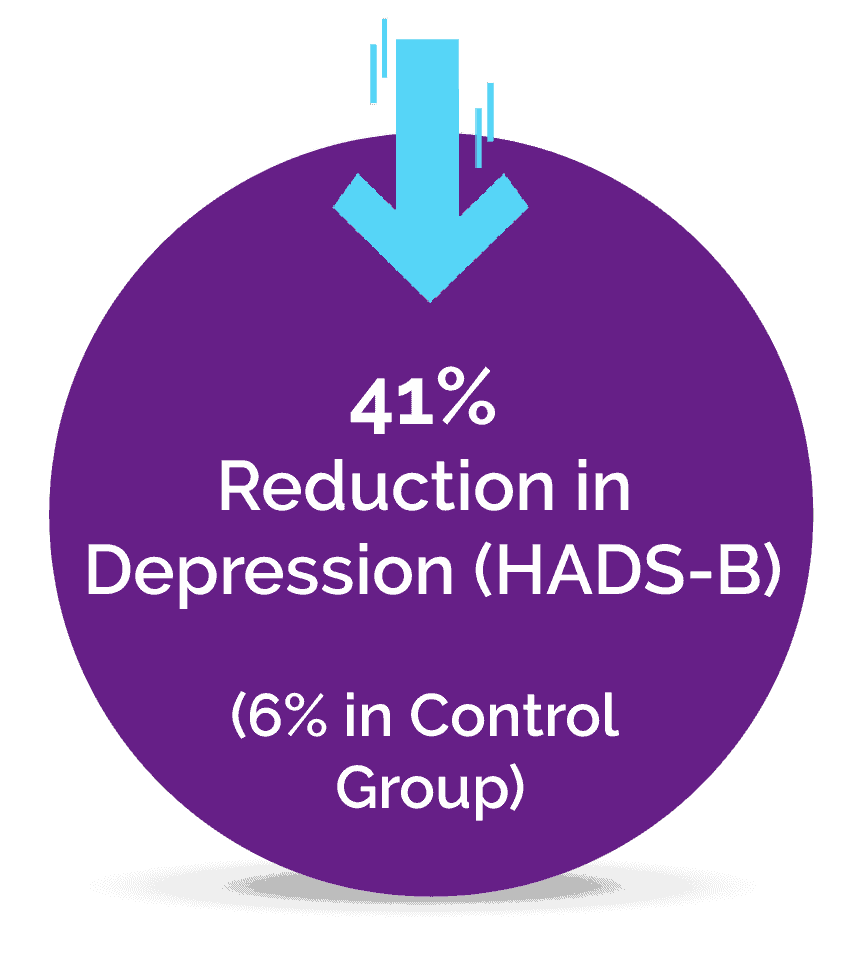
A Pilot Study Published in the Journal of Clinical Medicine
It is estimated that at least 2% to 5% of the population suffer from Fibromyalgia, a complex pain condition in some form, and until now there has been a lack of effective treatments. The novel Neuroplasticity program known as “Amygdala and Insula Retraining” was combined with Mindfulness to create 'MAIR', also known as the Gupta Program, and was tested for 8 weeks against a control group engaging in an equivalent amount of relaxation techniques.
The pilot study results found that after just an 8 week intervention, the MAIR group had significantly greater reductions in symptoms and pain, and increases in overall health, compared to the control group. These are the key results from the 8-week study: